- Physical Medicine Home
- Products
- Programs
- Applications
- Find a Clinic
- Blog
- Events
- Education & Training
- In The News
- Press Releases
System 4 Software v4.63
Legacy System 4 Software 4.63
| Overview | Details | Manuals |
Overview
Complimentary Software
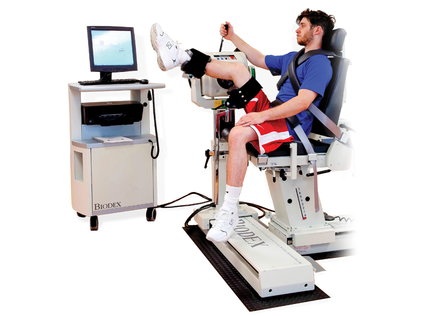
The v4.63 System 4 software update provides the Mixed H/Q Ratio Test and Multiple Angle Comparison Test for hamstring injury prevention and rehabilitation. The objective test protocols offered for the Biodex System 4 will provide valuable, isolated muscle-performance data. Test results, combined with established targeted outcomes, can be used for pre-emptive injury screening, managing rehabilitation and for determining readiness for return to play.
In addition, this important software update allows users to download all patient test information at one time and easily convert .csv files into a report format. It also includes expanded and updated normative data from pediatric through adult, ages 5-83.
*Requires Windows 7 Professional 64-bit OS. Hardware and software upgrades are available for earlier Biodex System 3 and 4 models. Contact Biodex Support Services for details, 1-800-224-6339 (int’l +1-631-924-9000).
Instructions:
To receive software update click here.
|
Optional program downloads, if required: |
|
| |
Details
System 4 Advantage Software 4.63 Supports Hamstring Protocols.
Hamstring injury is serious business. Regardless of whether you have an existing process
for protecting and strengthening hamstrings, the objective test protocols offered with the
Biodex System 4 will provide valuable, isolated muscle-performance data. Test results, combined with established targeted outcomes, can be used for pre-emptive injury screening, managing rehabilitation and determining readiness for return to play.
 Protocol #1:
Protocol #1:
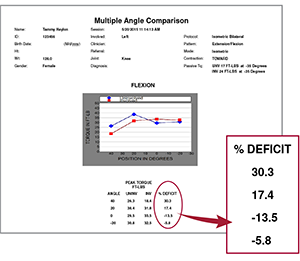
Multiple Angle Comparison Test
Examines isometric bilateral flexion peak
torque symmetry where the limb position
puts the hamstring in a lengthened
(stretched) state. Passive stretch is the
inherent force (or torque) produced by
the hamstring in the lengthened state.
When deficits are within 10%,
predisposition to re-injury is significantly reduced.
NOTE: This Protocol requires 830-550 Hamstring Attachment. Use of any attachment other than the 830-550, is outside proper and intended use.
Download Abstract:
(Paper in Review) ECCENTRIC STRENGTHENING AT LONG MUSCLE LENGTHS REDUCES HAMSTRING STRAIN RECURRENCES.
Tyler, et al; Orthopedic J Sports Med. Aug, 2014.
www.biodex.com/research/15151
| Have you considered adding lengthened state eccentric training to your current hamstring program? If so, check out this protocol: HAMSTRING INJURY REHABILITATION AND PREVENTION OF REINJURY USING LENGTHENED STATE ECCENTRIC TRAINING: A NEW CONCEPT www.biodex.com/protocol/15214 |
 Protocol #2:
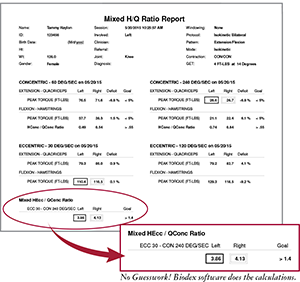
Protocol #2:
Mixed H/Q Ratio Test
Uses a ratio of eccentric and concentric flexion peak torque where, if the ratio exceeds 1.4, hamstring injury is greatly diminished.
NOTE: This Protocol utilizes standard Biodex Knee Attachment.
Download Study:
STRENGTH IMBALANCES AND PREVENTION OF HAMSTRING INJURY IN PROFESSIONAL SOCCER PLAYERS.
Croisier, et al; The American Journal of Sports Medicine. April 30, 2008.
www.biodex.com/research/15213
Manuals
Support
In The News
Biodex Medical Systems, Inc. devices help manage specific sports injuries and determine safe return to play
... read more >